Welcome to the June 2015 issue of FREYRFOREWORD!
A monthly round-up of the latest happenings and updates from Freyr.
Happy Reading!
DEVELOPING AND SUSTAINING RIGHT FIRST TIME ARTWORK CAPABILITIES
 Artwork design is an essential process in the supply of a pharmaceutical product which basically ensures patient safety while reducing recall risk.
Artwork design is an essential process in the supply of a pharmaceutical product which basically ensures patient safety while reducing recall risk.
LABELING: STRATEGIZE AND STREAMLINE PROCESSES TO MEET COMPLIANCE NORMS
 Labeling regulations keep changing and it’s especially critical for those industries where labeling and identifying parts and packages play a pivotal role in consumer’s safety.
Labeling regulations keep changing and it’s especially critical for those industries where labeling and identifying parts and packages play a pivotal role in consumer’s safety.
FREYR SUCCESS STORY
 Learn how Freyr helped a Global Top 5 Pharma Company in performing gap analysis across a large portfolio of 320 products.
Learn how Freyr helped a Global Top 5 Pharma Company in performing gap analysis across a large portfolio of 320 products.
FREYR SUCCESS STORY
 Learn how Freyr provided strategic submission and publishing services to a Global Top 20, $20+ Bn, Pharmaceutical Company.
Learn how Freyr provided strategic submission and publishing services to a Global Top 20, $20+ Bn, Pharmaceutical Company.
 |
||
| Artwork design is an essential process in the supply of a pharmaceutical product which basically ensures patient safety while reducing recall risk.
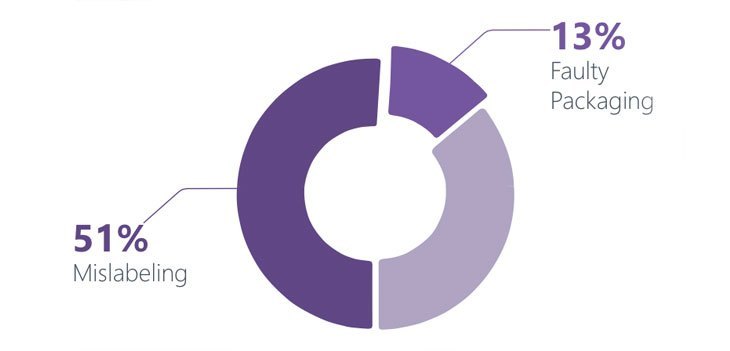
Artwork and labeling functions are an intrinsic part for the supply of a pharmaceutical product, and are under constant pressure to deliver an increasing number of projects in a compressed time frame. In the current business scenario, companies strive to differentiate themselves by reducing time to market, deliver quick product mix and quickly change products to market needs. Pharmaceutical and Life Science companies are governed by FDA validation, stringent quality norms and GMP. Miniscule errors can be to be costly, damaging and puts companies at risk owing to the threat of product recalls, health authority warnings and fines. The overall commercial success of a drug or pharmaceutical company is dependent on the right first time artwork, prior to entering into a new market. Costs associated with pharmaceutical packaging amounts up to 70% of the cost of the finished product. It is also reported that 35-40% of product recalls are attributed to packaging and labeling errors and omissions. There have been a total of 455 product recall notices out of which 51% were attributed to mislabeling and 13% because of faulty packaging, according to data covered over a six month period by the Food and Drug Administration (US FDA). GLOBALIZATION AND PHARMACEUTICAL COMPANIES: Owing to the expanding global markets, pharmaceutical companies must combat localization issues which include language, cultural and regulatory requirements. Emerging pharmaceutical markets also pose a challenge to the pharmaceutical companies which are trying to gain a foothold in the market. Delivering the Right First Time Artwork will enable Pharmaceutical companies to introduce new drugs and gain some ground in the new emerging market landscape. IMPORTANCE OF ARTWORK MANAGEMENT: SUPPORTING PRODUCT LAUNCH AND PATIENT SAFETY Producing artwork for the pharmaceutical is a significantly more disciplined process with the need for compliance, quality standards and validation which is of utmost importance. Pharmaceutical products can only be sold if they are packaged properly, shipping will only happen if the text on the packaging is absolutely correct. If incorrect text is printed on the product, the pharmaceutical company’s reputation and profit take a severe setback. Artwork as a function is critical as it enables a Pharma company in supporting product launch and patient safety. The function although does not offer a direct strategic competitive advantage however it offers the GxP attention as the efficacy of an active, packing line clearance and change control. Pharmaceutical product faces a tough test, and artwork design errors however minimal do have catastrophic results and also serve as the basis of the highest cause of product recall. Typically, artwork design involves coordinating information from many different sources. PRODUCT RECALL: AN INDUSTRY ESTIMATE
APPROACH: PROCESS IMPROVEMENT COMPLIMENTED BY TECHNOLOGY Artwork processes are still managed by manual process; Artwork management is nothing but a combination of good project management, repository management, version and label regulatory control and actual authoring process. Pharmaceutical companies must strategically build a streamlined process to avoid the perils of outsourcing to contractors which adds to compliance risks leading to fines, product recalls etc. ROBUST ARTWORK PROCESS: There has been a petition owing to which there have been innovations in technology, stringent quality control and the need to streamline systems for creating an accurate automated workflow process.
ARTWORK MANAGEMENT SOLUTION Streamlining artwork management processes has been the core focus of Pharmaceutical industry, which invests heavily in the packing every year. A trusted custom software solution has been the need of the hour, which can replace the manual process, which is prone to defects, delays in the design of lay outs, forms and approvals. An automated solution, aids in plugging loop-holes, closing the gaps and fast tracking the entire process right from design to print. COLLABORATION CHALLENGES Artwork and Labeling involves collaboration with various internal and external units. This includes
In addition, interaction with so many departments brings its own challenges
TECHNICAL CHALLENGES & POSSIBLE SOLUTIONS:
DEFINED ARTWORK MANAGEMENT PROCESS ASSURES RIGHT FIRST TIME ARTWORK Example model process with defined control points to ensure error free networks Create Source text→Define Change→Produce Artwork→Produce Printer Proof→Implement Make sure the text is right, proof read and verified for final printing Elements required for a successful artwork capability IMPROVEMENTS TO ARTWORK CAPABILITIES: A PHASED APPROACH Step 1: In Control Step 2: Efficient & Effective Step 3: World Class BUSINESS ADVANTAGES OF STREAMLINED ARTWORK FUNCTION A streamlined artwork function offers increased quality through reduction of product recalls or critical incidents & product launch due to Artwork errors. It also improves branding consistency among the artworks in terms of design, color and text while giving creditability to product and brand. As it will be audit and compliance driven – One process will be followed across the globe, thus reducing compliance driven penalties. In addition, it gives companies the opportunities to plan ahead around staffing and has measurable SLA’s around each step of the Artwork process. Furthermore, artworks can be reused for similar markets, thus significantly reducing time and cost; product launch schedule timelines can be planned accordingly. Overall Pharmaceutical companies can understand the triggers of artwork changes and business dynamics around it. OTHER BENEFITS
CONCLUSION Delivering quality artwork is a complex endeavor involving many moving parts in addition packaging and artwork presents a significant compliance risk. Artwork management is critical to delivery of a Pharmaceutical company’s business strategy. Right First Time Artwork can be achieved once the company undertakes the assessment of the current situation and base it against a comprehensive assessment model. A through gap analysis can also be done to understand key improvement areas across the organization. |
 |
||||
| Pharmaceutical and medical device industries are constantly changing with developing supply chain themes and technological advancements due to which labeling must be looked upon with a new perspective. A host of latest trends have compelled Pharmaceutical and medical device industries to rethink, strategize and streamline processes, meet compliance norms and start and learn best practices. Here are some of the latest trends in Labeling. | ||||
| IMPACT OF REGULATIONS ON LABELING Labeling regulations keep changing and it’s especially critical for those industries where labeling and identifying parts and packages play a pivotal role in consumer’s safety. In the medical device industry, companies now have to adhere to the changing norms/standards such as Unique Device Identification (UDI) and change the way products are labeled.
The pharmaceutical industry is making companies reconsider the way they do business owing to the ePedigree and the more current Drug Quality Security Act (DQSA). Companies must now get in line with standards and evolving regulations to change their supply-chain processes and adopt new labeling standards. Companies must either comply or face the consequences including hefty fines and loss of business. Some of the major regulations and standards in labeling are ePedigree In the US, most of the states have decreed pedigree requirement to protect consumers from contaminated medicine or counterfeit drugs. The law which was first initiated in the State of California seeks to track and serialize unit-level saleable packages (e.g; bottle of pills) throughout the supply chain. Bar code and RFID technologies are employed for implementing traceability. DQSA The law intends to strive for improvements in supply-chain efficiencies and control, as well as brand/product integrity. DQSA’s law establishes standards for interoperable exchange of transaction information, including documenting the history of product movement, among all trading partners using unique numerical identifiers for each unit of sale. GS1 The GS1 Systems of Standards offers global standards to fundamentally improve efficiencies and visibility of supply chain and applies to multiple industries ranging from healthcare to food and beverage and retail. To support safety initiatives bar codes are being implemented and to have a quick response to product recalls. GS1 also provides an EPCglobal (www.gs1.org/epcglobal) Drug Pedigree Standard and certification. UDI According to The Food and Drug Administration (FDA) final ruling, medical devices distributed in the US, must carry a unique device identifier. This includes Class III medical devices, which must meet UDI requirements and also include submission to the GUDID by September 24, 2014. A UDI system has the potential to improve the quality of information in medical device adverse event reports, better target recalls and improve patient safety. CENTRALIZATION: THE NEXT NORM IN LABELING In a bid to improve consistency and to streamline processes across their supply chains, many businesses are centralizing bar code labeling across all locations and geographies. Centralization supports business continuity and lowers the IT cost of maintaining multiple labeling systems. Owing to the recent innovation in enterprise labeling technology, it becomes imperative to move to a centralized labeling model. Here are three main reasons for centralization Labeling Consistency Organizations are trying to ensure their global locations produce labels in line with corporate standards with respect to formatting and data content perspective to improve supply chain efficiency. Marketing departments are becoming increasingly involved in making sure corporate brand standards are incorporated. Business Continuity Global organizations can swiftly shift labeling to support operations and removes risk in replicating labels which may be facility or region specific thereby supporting business continuity in the event of natural disasters and geopolitical unrest. IT Maintenance Costs Managing multiple, different labeling solutions across global operations becomes increasingly difficult. However a single, centralized solution can be deployed by organizations to reduce IT maintenance costs while allowing businesses to meet customer and regulatory labeling requirements. |
||||
| CUSTOMER RESPONSIVENESS : ADHERENCE TO LABELING REQUIREMENT Suppliers and partners must be flexible to meet unique customer labeling requirements to show customer responsiveness. Customers are now seeking providers to meet their own labeling requirements for data content, images, symbologies and languages. Advances in enterprise labeling have made sure companies gain new customers while catering to the needs of the existing customers. Customer responsiveness in labeling must ideally meet the following expectations:
Specific Data Content Inclusion Unique data attributes are now sought by customers on labels provided by their suppliers in a bid to streamline their processes and limit relabeling when goods are received. The unique data attributes include transactional data like quantities, lot numbers or expiration dates to actual data from the customer’s enterprise applications including product codes or purchase order numbers. Formatting Standards Customers are looking to get their preferred label format delivered by suppliers; this desire is stoked with an aim to control label formatting across multiple suppliers. Also customers want to maintain brand consistency and simplify the receipt of goods and are specifying where data elements appear, images to be used and incorporation of bar code symbologies. Once these are incorporated, customers are satisfied to receive goods that align with their internal labeling standards. Regulations Customers want their supplier and partners to include the necessary data in the appropriate language for subsequent local processing. In addition, the ever changing standards/regulations dictate which language needs to be applied to labels where goods are going through their supply chain. LABELING: DATA-DRIVEN AND INTEGRATED Critical need for label accuracy compels the adoption of automated, transactional-based labeling by companies to improve operational efficiency. A data-driven approach will help companies replace static and dynamic label templates which can simplify maintenance and ensure accuracy. The move towards data-driven labeling is because of Integrated Labeling Most companies are replacing manual labeling processes with automated, integrated labeling which is the best practice of initiating labeling from their transactional system. Importance of Big Data Data-driven approach to labeling enables support for all labeling needs by making fields on a label dynamic and variable. Using a single update a change can be initiated to all labels without making changes to numerous templates. Integration: Product Lifecycle Management and Content Management Systems Product Lifecycle Management (PLM) and Content Management Systems (CMC) are the main definitive sources of data which can appear on a label. Data-driven integration with an enterprise labeling system is paramount and by taking content from PLM and CMS, companies can ensure that label content is accurate and up-to-date. ROLE OF SUPPLIERS Increasingly companies are looking towards suppliers and partners to be meet their labeling requirements thereby increasing supply chain collaboration and streamlining operations. This will lead to accuracy and immediate deployment of labels. Relabeling Costs Companies must ensure that suppliers adhere to corporate labeling standards which allow compliance and costly relabeling work is avoided. Although relabeling process ensures proper bar code symbologies, images, branding and data content to support subsequent processing, it is a very costly process. Supply-Chain Association Forecasting, inventory level management and on-time delivery rates- technology is leveraged by customers, suppliers and partners to show tangible benefits. Supply-chain collaboration is increasingly becoming important. Labeling Errors Reduction Enterprise labeling technology can help companies to combat supplier label change errors by making them use their labeling solution to print accurate and current labels in line with labeling standards. |
 |
||||||||||||
| BENEFIT HIGHLIGHTS
TECHNOLOGICAL ENVIRONMENT MS Office (MS Visio, MS Excel, MS Word), SharePoint, Citrix BUSINESS IMPERATIVES The company is known popularly for its cosmetics and baby care products. Freyr is associated with the client’s Global Labeling Management and related services like:
CHALLENGES
FREYR SOLUTIONS & SERVICES
CLIENT BENEFITS
|
||||||||||||
 |
||||||||||||||
| BENEFIT HIGHLIGHTS
BUSINESS IMPERATIVES
CHALLENGES
FREYR SOLUTIONS & SERVICES
CLIENT BENEFITS
|
||||||||||||||