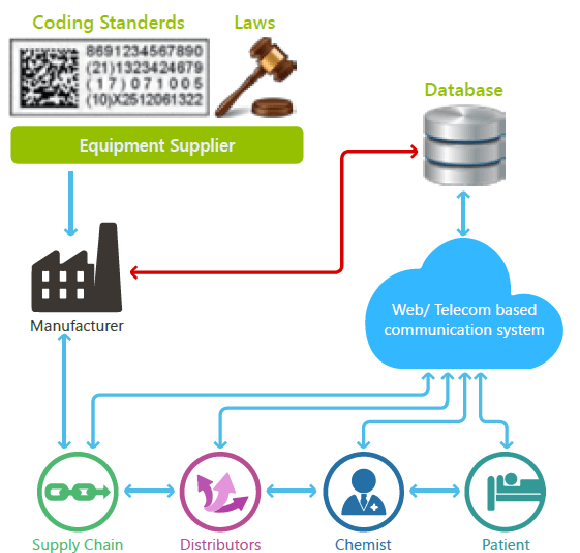
| SERIALIZATION
It refers to the allocation and placement of unique markings on a primary package. The markings can be a two-dimensional or RSS bar code, a human readable letter/number code or unique serialized codes that can be written onto a radio-frequency identification (RFID) tag/label. Variable data printers or preprinted labels or cartons are employed to position unique codes on each package which are then read by a vision system. Furthermore, the unique codes are uploaded to an event repository database which can be accessed by various parties, including pharmacists, law enforcement officials and even consumers, once the product is shipped and sold.
The individual packages will have unique codes which can be grouped/electronically linked to a shipping case and even to a pallet and other levels of packaging which in turn creates a child/parent/ grandparent relationship. As a result of the grouping, if the bar code on a pallet is scanned at a warehouse, the brand owner or trading partner will have tracking information regarding all shipping containers and primary containers at that warehouse. In addition, once an ePedigree law comes into action, serialization and aggregation will help to track-and-trace products from the point of packaging to the pharmacy or healthcare facility.
CURRENT SITUATION:
Serialization requirements are in heterogeneous stages of development in the US, Canada, EU and in its member nations as well as in Turkey, India, China, Brazil, Argentina and South Korea. Despite the variations found in countries laws, each nation’s regulations tend to be built around GS1 standards and are quite similar. Although the GS1 format is the most desirable standard, International Organization for Standardization (ISO), Internet Engineering Task Force (IETF) and other competing standards also apply to serialization. All activities related to drug serialization that are evolving in different countries are backed up by the overarching global initiative conducted on the World Health Organization (WHO) level. The WHO set up the International Medical Products Anti- Counterfeiting Taskforce (IMPACT).
Drug Supply Chain Security Act (DSCSA) Standards for the Interoperable Exchange of Information for Tracing of Certain Human, Finished, Prescription Drugs: How to Exchange Product Tracing Information, released by FDA on 28 November 2014, is meant to address: How information is exchanged between entities within the pharmaceutical supply chain.
DSCSA Implementation: Annual Reporting by Prescription Drug Wholesale Distributors and Third-Party Logistics Providers was released on 8 December 2014 and explains how wholesalers and third-party logistics providers (3PLs) should report DSCSA information to FDA on an annual basis. Argentina legislation is effective but limited to certain products; however the number of products falling under this legislation is rapidly growing.
In January 2014, ANMAT has published a detailed specification of the central Argentina database (trazabilidat) in Spanish. In December, Brazil published RDC 54/2013, specifying track and trace requirements, accordingly manufacturers must provide serialization and tracking data for three batches of products by 10 December 2015. All pharmaceuticals must be serialized and tracked by 10 December 2016. For a growing market, that’s a critical milestone. On 23 January 2014, the first public hearing discussing implementation was held. Plenty of other countries are preparing for legislation Saudi Arabia by March 2016, Jordan and Ukraine by 2017 including Columbia and Mexico.
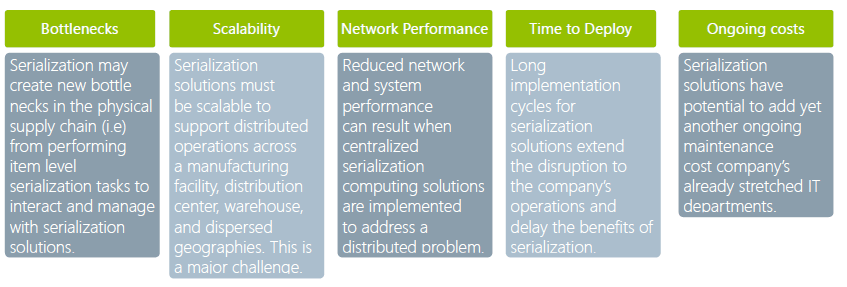
POSSIBLE STUMBLING BLOCKS IN IMPLEMENTATION
BENEFITS:
The leading companies are demanding solutions that will not only please legislative mandates, but also help them in achieving business benefits beyond compliance. These benefits come from the compact product control that serialization provides. They include:
- Superior product authentication and integrity, which will protect and enhance the company’s
- brands and shareholder value
- Pronounced revenue share by reducing the “gray market” activity that occurs when products are
- counterfeited and diverted.
- Finer control over and visibility into the supply chains, leading to accurate shipments.
- Fast and efficient reverse logistics or recall processes.
- Serialization also provides huge benefits for clinical trials as they can be conducted, reported and analyzed more efficiently if each of the unit doses being taken in the trial are serialized
- The healthcare industry can benefit from serialization of medical devices as well
- Link between the brand owner and the consumer can be used for creative brand loyalty programs
- (as marketing tool)
- Companies, in countries with single-payer or other centralized health care systems, reap additional
- benefits through improved ability to recoup payment from centralized government agencies that reimburse patients drug costs
|
 Cosmetic products have an estimated worth of €67bn in Europe, which is regarded as a massive enterprise. The primary requirement during the development of a cosmetic product is to ensure protection for the user’s health which is also the basis of the cosmetic legislation.
Cosmetic products have an estimated worth of €67bn in Europe, which is regarded as a massive enterprise. The primary requirement during the development of a cosmetic product is to ensure protection for the user’s health which is also the basis of the cosmetic legislation. Counterfeiting, theft, diversion and false returns to manufacturers are few of the problems faced by the pharmaceutical industry across the globe. According to the World Health Organization (WHO), counterfeit drugs make up 1% of the supply in developed countries (including millions of prescriptions in the US alone) and 30-40% in developing countries.
Counterfeiting, theft, diversion and false returns to manufacturers are few of the problems faced by the pharmaceutical industry across the globe. According to the World Health Organization (WHO), counterfeit drugs make up 1% of the supply in developed countries (including millions of prescriptions in the US alone) and 30-40% in developing countries.  In 2011-12, the Australian Pharmaceutical industry clocked in exports of $4.06 billion. In the same year, the industry collected around $6.6 billion from Pharmaceutical Benefits Scheme (PBS) sales.
In 2011-12, the Australian Pharmaceutical industry clocked in exports of $4.06 billion. In the same year, the industry collected around $6.6 billion from Pharmaceutical Benefits Scheme (PBS) sales. Our latest new client wins are a fantastic fit for our regulatory expertise and services, we’re absolutely delighted to be working on these projects for one of the Top Global Pharmaceutical Companies.
Our latest new client wins are a fantastic fit for our regulatory expertise and services, we’re absolutely delighted to be working on these projects for one of the Top Global Pharmaceutical Companies. Learn how Freyr secured significant cost benefits to a Global Top 5, $70+ Bn, Pharma & Consumer Healthcare Company while offering strategic advice on claim support for “to-be” marketed products.
Learn how Freyr secured significant cost benefits to a Global Top 5, $70+ Bn, Pharma & Consumer Healthcare Company while offering strategic advice on claim support for “to-be” marketed products. Freyr was engaged by a Global Top 5, $70+ Bn, Pharma & Consumer Healthcare Company for master dossier creation for future markets which incurred 40% savings on cost of compliance.
Freyr was engaged by a Global Top 5, $70+ Bn, Pharma & Consumer Healthcare Company for master dossier creation for future markets which incurred 40% savings on cost of compliance.