SOP Writing and SOP Review Services - Overview
The world of life sciences is moving towards procedural standardization, risk-based methodology, Quality by Design (QbD), and a common control framework. However, Standard Operating Procedures (SOP) review and SOP writing services may sometimes require organizations to invest a lot of time and money to research global legislation and to update the systems perennially. In such scenarios, organizations look for expert SOP writing services and SOP review services.
Freyr can provide SOP authoring services for all types of pharmaceutical practices, research and development, Annual Product Quality Review (APQR), information technology Regulatory Affairs, quality management review, clinical research, and all categories of pharmaceutical SOPs.
With a comprehensive knowledge of new SOP writing/SOP review or to amend/standardize existing ones, Freyr, with the help of its expert team, offers end-to-end SOP writing services and SOP review services that span across:
|
SOP Writing Services |
SOP Review Services |
SOP Integration Services |
|
|
|
|
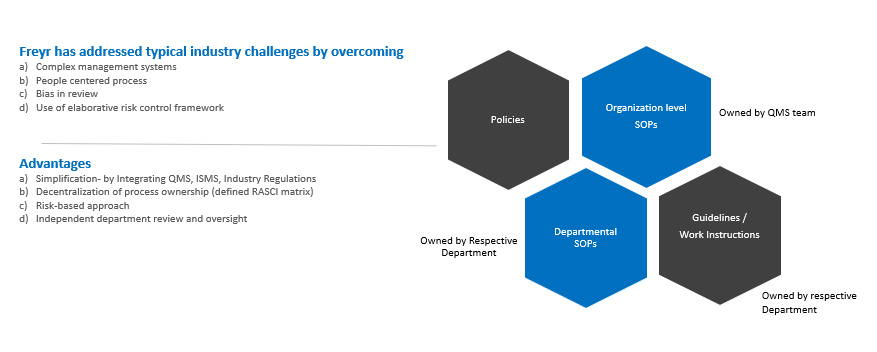
Freyr offers an integrated process of multiple management systems (ISO 9001, ISO 27001 with USFDA, EMEA, and MHLW) |
||
SOP Writing and SOP Review Services - Freyr Expertise
SOP Gap Analysis
Freyr has been providing SOP writing services and SOP review services for the pharma, healthcare, and biotechnology industries. In the last five (05) years, Freyr’s Compliance & Validation Centre of Excellence (CoE) has created/reviewed/harmonized five thousand and five hundred (5500)+ SOPs for its customers and continues to support them.
Following the risk-based approach and common control framework methodologies, Freyr supports organizations with integrated SOPs catering to all Regulatory requirements. Freyr’s expert group of QMS remediation can establish process architecture by integration of multiple regulations for unified management systems (ISO 9001, ISO 27001, GLP – 17025, Medical Laboratories – 15189, CAP with USFDA, EMEA, MHLW, WHO, GxP, and ICH).
- Process designing.
- Establishing process architecture.
- Risk-based approach.
- Common control framework.
- Integration of multiple regulations for unified management systems.
Additionally, Freyr has catered audit and audit readiness support (including SOP writing) for twenty (20)+ large/medium/small pharma companies with global Health Authorities (HAs), including the US FDA, MHRA, EMA, CDSCO, ANVISA, SFDA, HALMED, NPRA, HSA, and Health Canada.
Process Approach for Updating and Creating SOPs
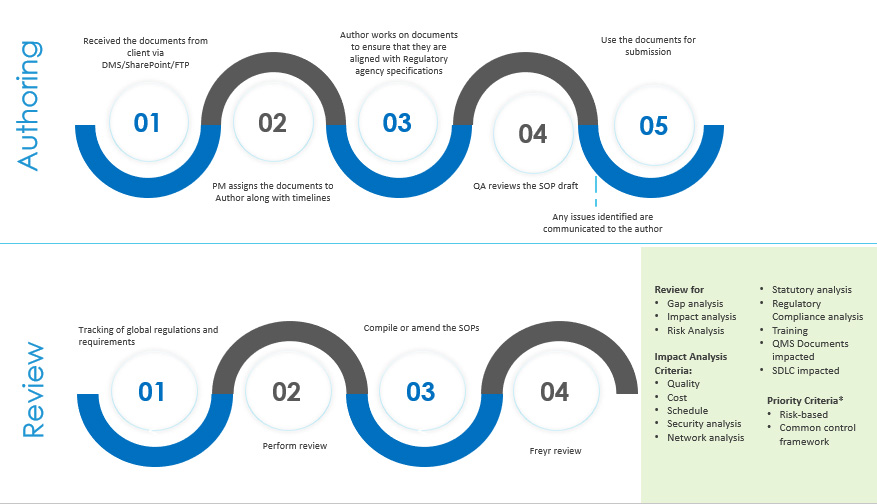
SOP Authoring and Review
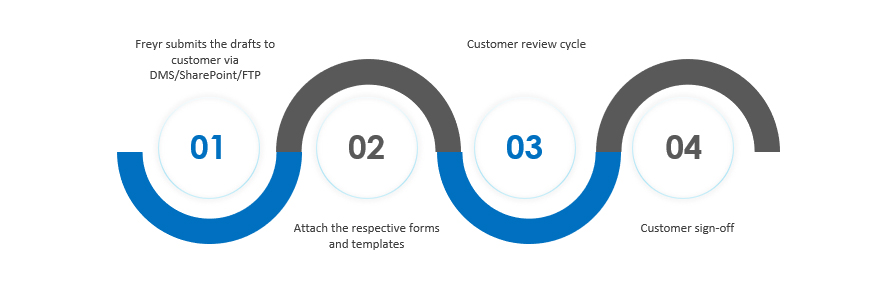
Customer Review and Sign Off
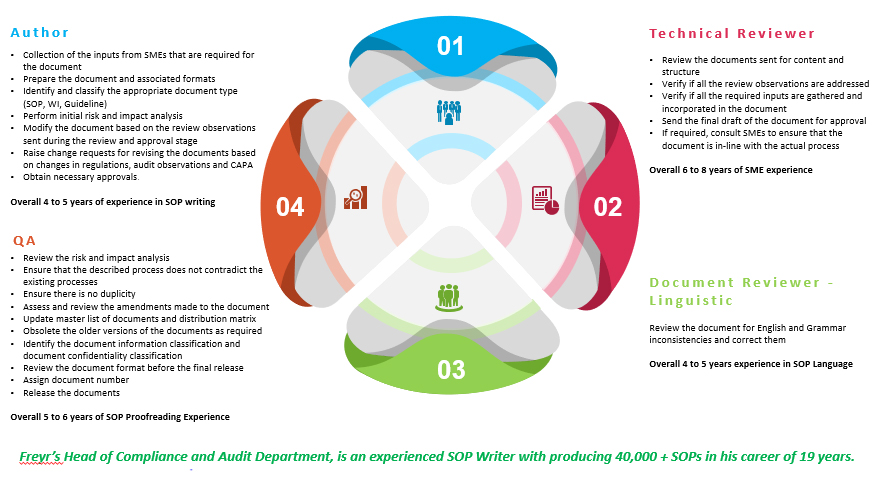
Overview of the SOP writing department, Compliance & Validation
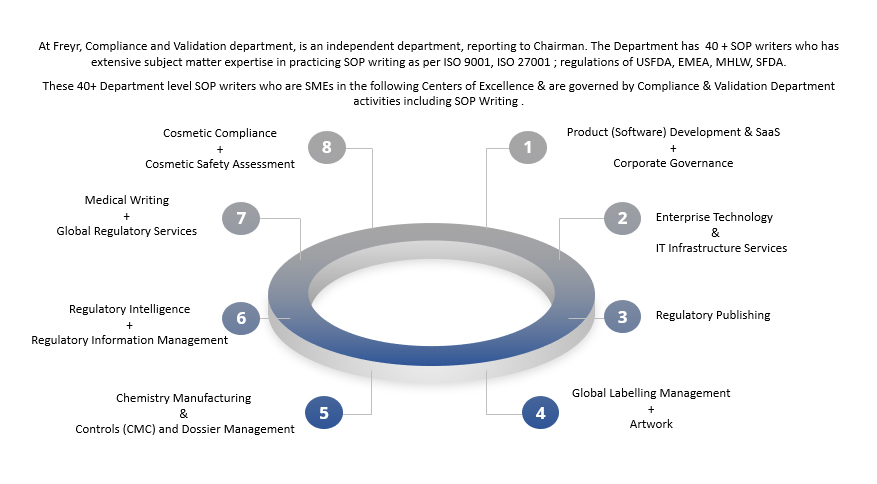
Level & Experience at Freyr for SOP writing
SOP Review Services
While expanding businesses to newer markets, life sciences organizations are required to develop new SOPs based on the regional Regulatory requirements or must update/upgrade the existing processes to align them with new demands. But before SOP preparation, the right approach is to identify the gaps between processes and remediate them accordingly.
Freyr is experienced in a range of projects on SOP review services. The simplest part of the service offering is to review existing SOPs for accuracy and provide gap analysis reports to complex projects, which involve SOP review and remediation, resulting in SOP standardization, rationalization, or SOP optimization.
SOP Standardization
As the business grows, the standards to be followed or adhered to will also increase. There will be times when a customer has several standards and regulations to follow, which may include SOPs for safety in the pharmaceutical industry. In such scenarios, maintaining and following different streams of SOPs for each standard will double up the procedural complexities for companies. The need of the hour is to unify or standardize the SOPs.
SOP Rationalization
At times, organizations create SOPs to let go of the audit. But there is no use implementing these. Either they are not followed by employees or they could make processes complex if integrated. To avoid such situations, it is necessary to rationalize the SOPs that bring some value to the organization.
Freyr provides SOP review services and rationalizes them in addition to adhering to applicable regulations and assists organizations to succeed in SOP compliance audits, thus adding real value to the company.
SOP Reconciliation and SOP Optimization
Though there is no set guideline, SOPs must be reviewed at defined and periodic intervals as a general practice. With different formats and versions accumulated, there is always a chance for overlapping and duplication. During such times, organizations must reconcile their SOPs, while continually improving processes to support their growth and business.
With a clear-cut knowledge of SOPs and quality systems, Freyr assists organizations to reconcile and optimize the SOPs periodically. Freyr’s expert compliance team can monitor and carry out incremental updates.
SOP Integration
Mergers and acquisitions are an integral part of the current business scenario. But when they take place, bringing together and managing the procedures, standards, SOPs, and quality systems of both organizations could be grueling. In addition to aligning with the existing standards, setting up SOPs for the newly formed organization will be even more hectic, especially when it is to come into existence within a short turnaround time.
With an in-house compliance team, Freyr offers end-to-end SOP integration services to analyze the gaps between both organizations’ systems, standardize existing systems, and design new SOPs when needed. Freyr’s experts extend system integration support for designing seamless processes and workflows.
Freyr Advantages
- Proposing cost-effective process options without compromising the objective of product quality, Regulatory compliance, patient safety, data integrity and security
- Integrated process models
- Proven practicable validation and qualification strategies
- Quick turnaround time
