Overview
As per the United States Food and Drug Administration (USFDA) and Health Canada norms, adopting technology, its processing, managing, labeling, and details of any label changes made, including changing the content of the formatted label and the carton labeling or container labeling, have to be electronically submitted using the Structured Product Labeling (SPL services) and Structure Product Monograph (SPM services).
Structured Product Label (SPL)
Structured Product Labeling (SPL) is a Health Level Seven (HL7) standard based on Clinical Document Architecture and the HL7 Reference Information Model (RIM) accredited by the American National Standards Institute (ANSI) for the exchange of product information. Structured Product Labeling or SPL documents include a header and body. The header includes information about the document such as the type of product, its author, and details about its versioning. The document's body includes product information in both structured text and data element formats. The FDA uses SPL documents to exchange information elaborating upon various product-related topics.
Structured Product Monograph (SPM)
In 2016, Health Canada announced that the SPL would be considered a Structured Product Monograph (SPM), and it should be submitted in an electronic format. Ensuring the same, the Agency has released guidelines and made it a mandate to submit SPM by Spring 2021. Health Canada adopted the HL7 Structured Product Labeling (SPL) standard for its product monograph submissions and has even developed pilot projects.
To assist Life Sciences organizations, Freyr provides SPL services and SPM services by integrating a web-based tool that automates compliance with the current SPL and PLR standards as established by the FDA and SPM standards for SPL submissions established by the Health Canada in Health Canada product monographs template. Freyr has already started working with Health Canada on several pilot SPMs and ensured all controlled vocabularies and validations have been included in the tool. Currently, Freyr’s SPL services and SPM services support six (06) of the templates which have been released by HC with a web-based, comfortable interface that does not require any add-ons.
The SPL Submission Process:
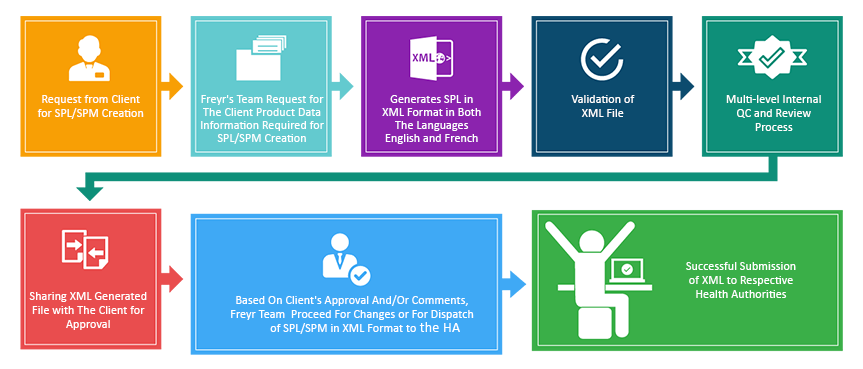
SPL/SPM Submissions – Freyr Expertise
- Comprehensive SPL services by providing SPL submission/SPM conversion services to handle all types of products, including prescription drugs, OTC products, scheduled drugs, veterinary medicine, homeopathic products, and bulk ingredients, as well as experts to dispatch and publish to the Health Authority.
- Implementation of all templates with controlled vocabulary released by the respective Health Agencies.
- Managing, recognizing, and resolving the errors made while publishing both Structured Product Labeling (SPL)/Structured Product Monograph (SPM) formats.
- Tackling registrations like NDC labeler code request SPL, bulk ingredients & establishment registration, etc.
- Drug labeling, listing, and other drug product submissions.
- Content-of-labeling for prescriptions (Human and Animal) drug labels, OTC, and BLAs.
- SPL services and SPM services include Validation of SPM/SPL submissions and successful publishing of the error-free labeling content as per the Health Authority guidelines.
- Identifying and defining a flexible delivery plan that can withstand last-minute updates
- SPM services Conversions of both French and English formats for SPM
- Comparison of texts in two (02) different versions to match the label documents
SPL/SPM Submissions – Freyr Advantages
- A project brief that clearly outlines the individual roles and responsibilities, dependencies, and other critical aspects for project success.
- Communicating project expectations both at the beginning of the project during the “starting up” project phase and at regular intervals during the delivery phase, as well as periodically during the delivery phase.
- Continuous project monitoring and immediate resource ramp-up from the rolling bench.
- Proactive training and system access for resources on the bench.
Ensure Consistent and Reliable Product Labeling Information for Efficient SPL-SPM Submissions
Structured Product Labeling (SPL) & Structure Product Monograph (SPM) is the mandatory document markup standard for Regulatory submission of label content, product and facility information, and any subsequent changes to an existing label information in an electronic format. SPL-SPM format defines the structure and content of label information as required by the United States Food and Drug Administration (US FDA) and Health Canada. The US FDA has mandated SPL requirements for over 50 types of documents, and HC has mandated SPM requirements for over 6 types of documents.
Would you like to decode complete information and gain a first-hand experience of Freyr SPL-SPM?

